The Benefits of Molecular Testing for Gastrointestinal Parasites
Advancing diagnostics for GI parasites
Multiplex PCR assays for detecting parasites have many advantages compared to traditional testing, such as stool ova and parasite microscopic exams
- Provides a faster, automated process to improve laboratory throughput
- Rapid high throughput screening for the majority of the clinically relevant parasites in the one sample
- Allows experienced FTEs to be redeployed to support other laboratory testing needs
- Increased sensitivity and specificity to support improved positive detection rate
- Supports the detection of parasites not easily detected by traditional methods (e.g. Cyclospora spp.)
- Molecular solutions are more accurate compared to stool ova and parasite microscopic exams which requires highly trained staff and human judgment
- Molecular solutions are easy to use and require minimal technical experience
- Improves reporting and surveillance for epidemiological studies
- Improves turn around time (TAT) to support clinicians to provide rapid and appropriate administration of anti-protozoal therapies to improve patient management
- Reduced downstream costs associated with pathology services or hospitalisation
Hear Professor Marc Couturier discuss how molecular ova and parasite testing has supported his laboratory in the on-demand webinar: The burden of gastrointestinal parasites and advances in ova and parasite diagnostic screening. Click here
"Molecular detection for common gastrointestinal protozoa is the logical progression of testing, especially given the ever-increasing volumes of traditional O&P testing and the decreasing workforce and proficiency labs are experiencing"
Prof. Marc Couturier, Medical Director, ARUP Laboratories
How Does Molecular Parasite Testing Work?
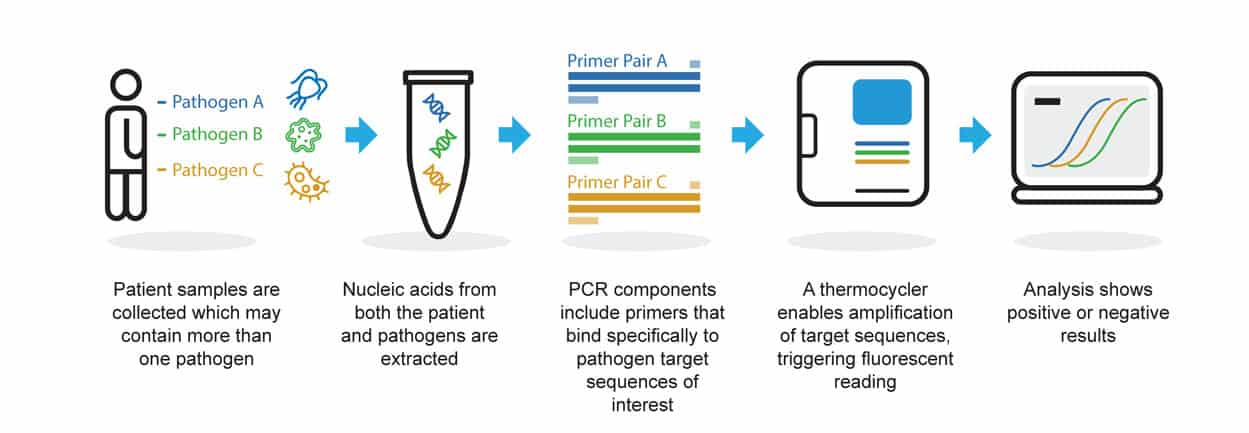
Molecular testing for gastrointestinal parasites is an application of real-time multiplex PCR developed by Genetic Signatures, driven by our proprietary 3baseTM technology. This technology improves the efficiency, sensitivity and specificity of multiplex PCR. Learn more about the advantages of 3baseTM here.
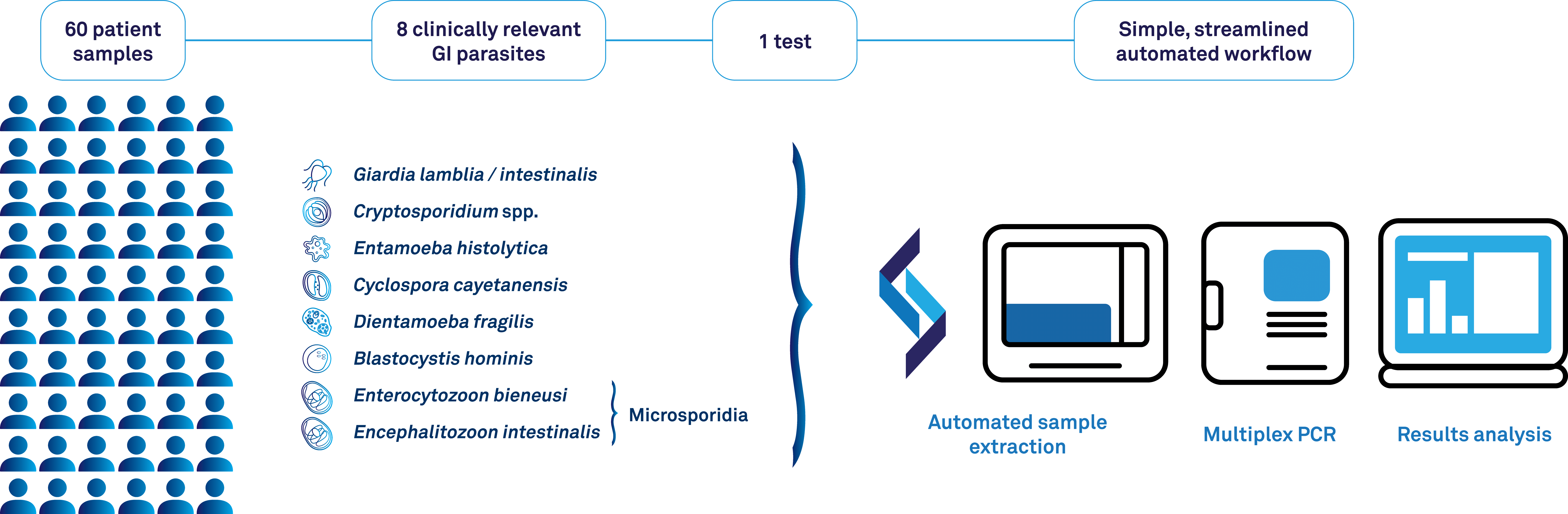
The EasyScreen™ Gastrointestinal Parasite Detection Kit allows patient samples to be tested for 8 clinically relevant gastrointestinal protozoa pathogens at one time. Using the process of PCR, unique target genes for each organism are detected, outlined in the diagram below.
Genetic Signatures’ EasyScreenTM Gastrointestinal Parasite Detection Kit has been cleared for sale by the FDA in the United States.
Contact us for more information on the regulatory status of our products in your region. When using our products always use the label and follow the directions for use.
Syndromic testing for 8 gastrointestinal parasites in a single test
Up to 60 patients screened in a single, automated workflow...with same day reporting!