In Vitro Diagnostic Regulation (IVDR)
IVDR
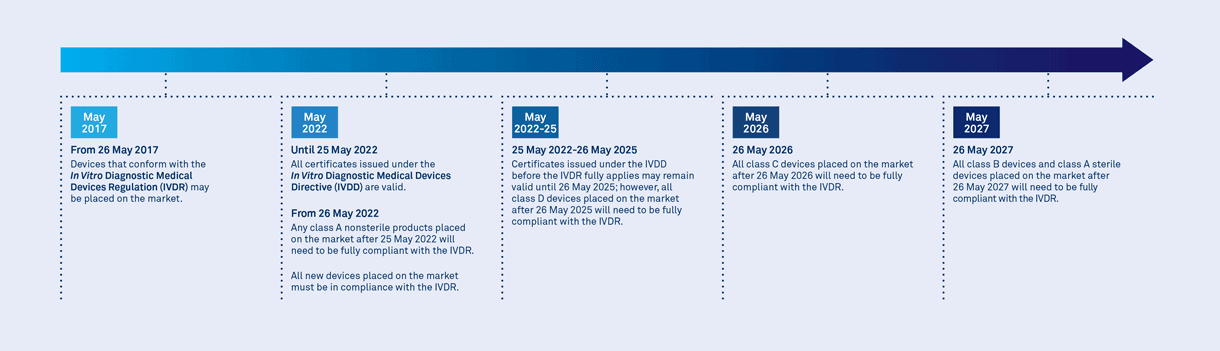
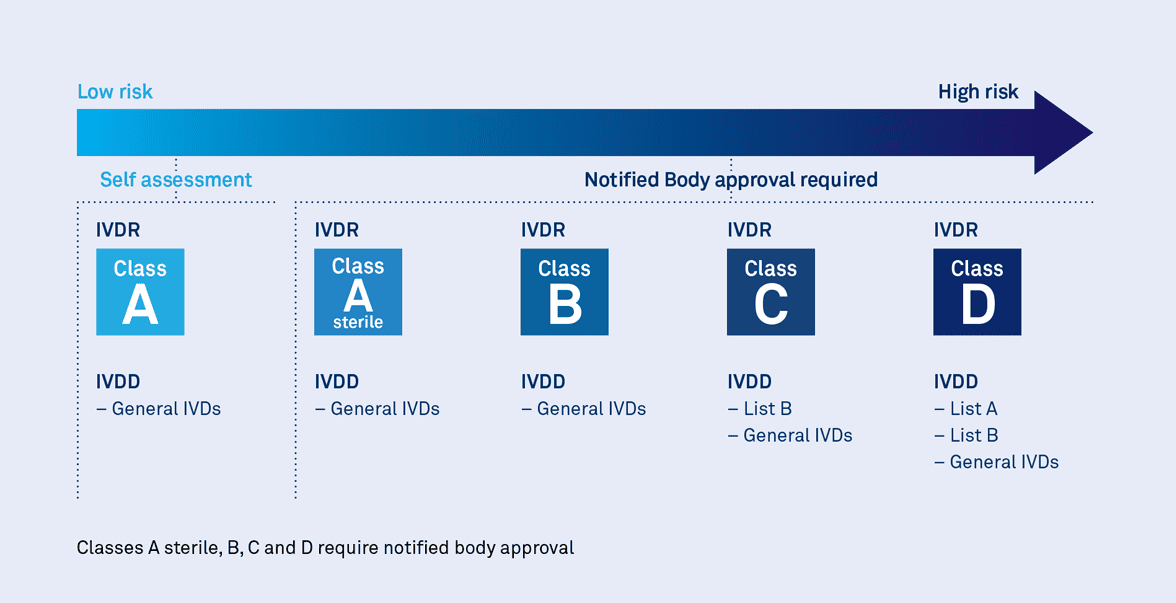
The In Vitro Diagnostic Regulation (IVDR) 2017/746 governs the safety and performance of in vitro diagnostics (IVDs) across their lifetime. The IVDR was adopted in 2017 and came into effect on May 26, 2022, replacing the previous In Vitro Diagnostic Directive (IVDD). The regulation aims to enhance the safety, performance, and reliability of IVDs while ensuring their proper assessment and conformity before they are placed on the market.
Compliance with the IVDR is mandatory for IVD manufacturers who wish to place their products on the European market. The regulation aims to enhance patient safety, improve the quality of IVDs, and promote innovation in the field of in vitro diagnostics.
Learn more about IVDR from the resources below:

IVDR Transition Timelines
Learn more about how Genetic Signatures can support you through the IVDR transition
Genetic Signatures IVDR transition
As part of the Genetic Signatures IVDR upgrade, the IVDR requirements have been embedded in our Quality Management System (QMS). Our Notified Body (NB 2797) has confirmed that our QMS meets the requirements of IVDR 2017/746 Annex IX Chapter I and III (Quality Management System).
Genetic Signature's EasyScreen™ Sample Processing Kits, Detection Kits and instruments are all within the scope of the transition to the IVDR.
The sample preparation is IVDR compliant and available now. EasyScreen™ Detection Kits which are IVDD compliant are also available now.
We are currently working to ensure Class B, C and D products are IVDR compliant according to the transitional periods. Genetic Signatures will continue to ensure we meet the IVDR needs of our customers.
IVDR FAQs
Contact Us
Contact our team for more information on our products and the regulatory status in your region