Sample preparation & PCR Set-up
Simple Sample Processing
- Universal extraction kit for all sample types
- The EasyScreen™ Sample Processing Kit offer streamlined preparation of nucleic acids directly from the primary specimen
- The kits isolate both DNA and RNA and are compatible with all EasyScreen™ Pathogen Detection Reagents
Explore our automated system for sample extraction and PCR setup, validated with the use of EasyScreen™ Sample Processing Kits:
GS1
- Performs both sample extraction and PCR setup
- Automated sample processing and PCR setup
- 384-well PCR setup on one platform
- Wizard-driven interface enables onboard sample and reagent traceability, as well as supporting minimal wastage
- Automated data analysis and integration to Laboratory Information System (LIS)
- Single platform with small footprint improves use of space and has cost-associated benefits
- Speak to our team about PCR system compatibility
For more information contact your local representative or fill out our contact form and we will be in touch.
Real-Time PCR Set-up
Once samples are prepared using the EasyScreen™ Sample Processing Kit any (or all) of the applicable EasyScreen™ Pathogen Detection assays can be set up as per the Real-Time PCR workflow using our Genetic Signatures automated workflow solutions which combines both sample extraction and PCR set-up.
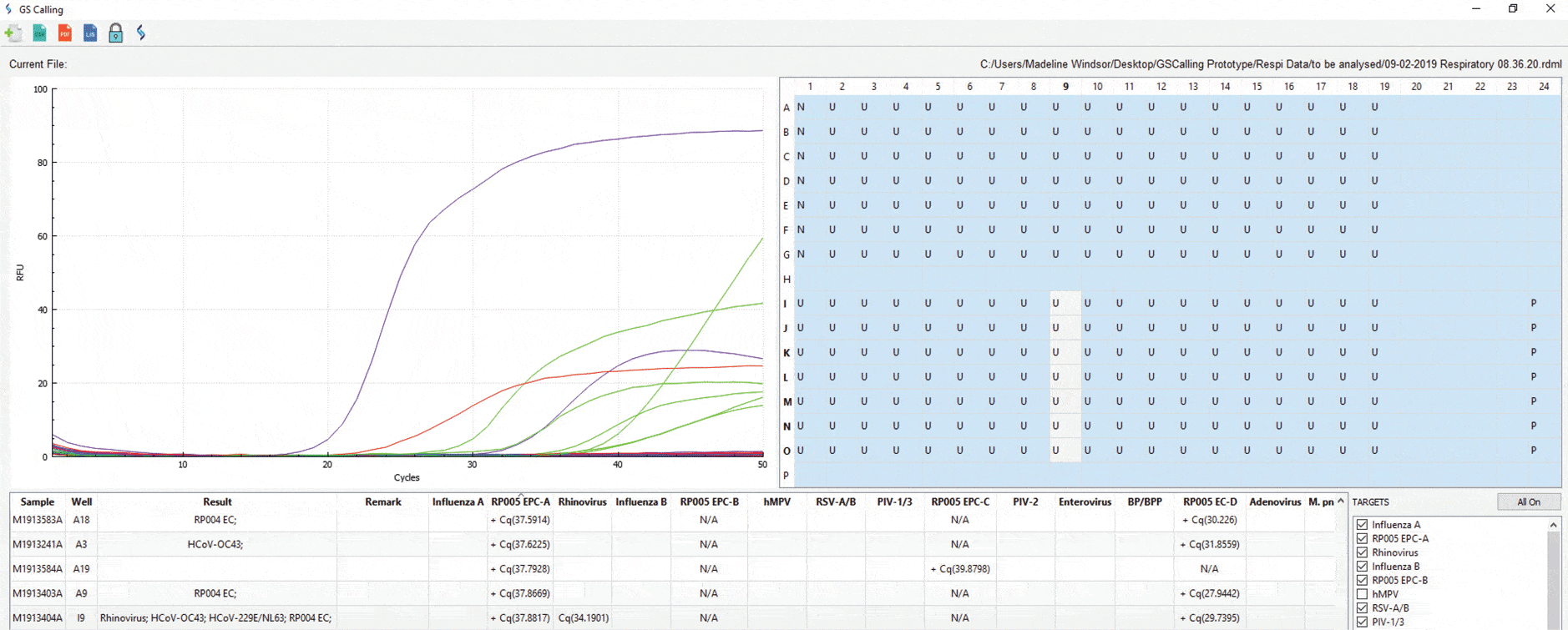
Genetic Signatures automated calling software: GS-Call
- Easy to interpret calling software
- Integrate into laboratory LIS/LIMS
- The flexibility of selecting/deselecting specified target
- Print reports in PDF format
- Validated on Bio-Rad CFX
Regulatory
- EasyScreenTM Sample Processing Kits SP008B & SP012 are FDA-listed clinical concentrators
- EasyScreenTM Gastrointestinal Parasite Detection Kit and GS1 workflow is FDA (510k) cleared for sale in the United States
- EasyScreenTM ASR solutions are for research use only and are not available for in vitro diagnostic use in the United States.
Contact us for more information regarding the specific conditions for each Automated Nucleic Acid Extraction and Real-Time PCR Platform.
