Streamlined & simplified molecular testing for gastrointestinal parasites
A comprehensive FDA 510(k) cleared solution, uniquely differentiated with 3base™ technology
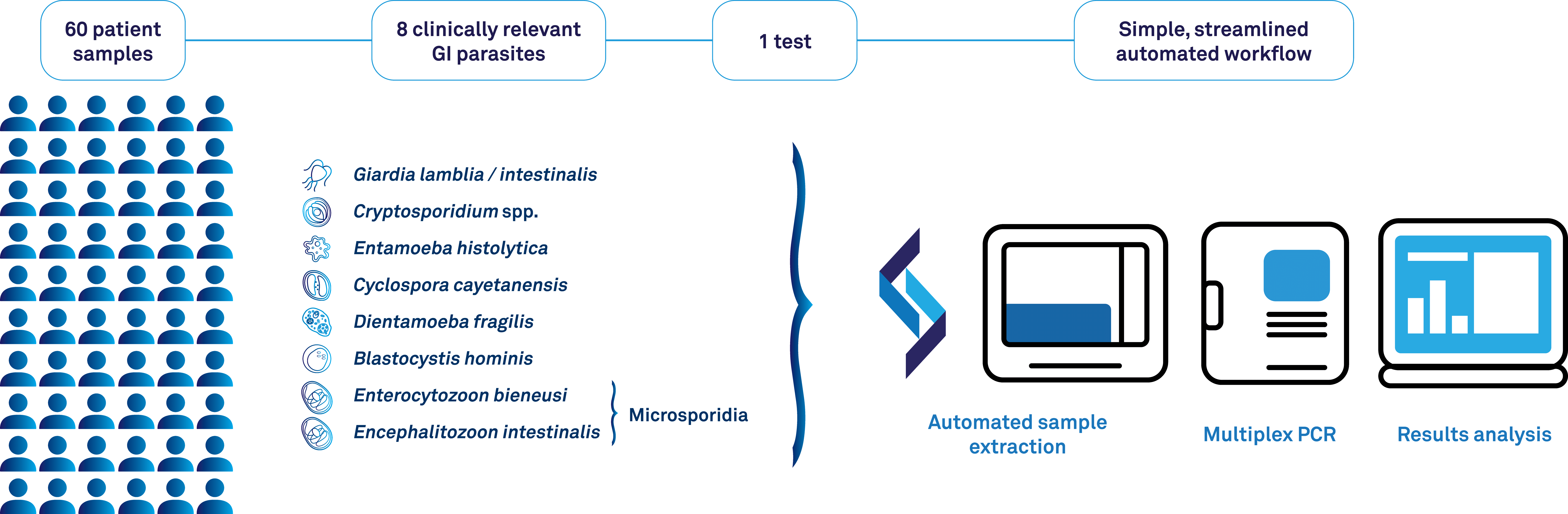
Detect 8 parasites in a single test

The Need for Molecular Testing for Gastrointestinal Parasites
Each year, over 3.5 billion people worldwide are infected with GI parasites, resulting in over 200,000 deaths and significant health and economic burdens(1). It is estimated that there are approximately 65 million cases of parasitic GI infections per annum in the US(2-6) with only 15% presenting to medical professionals(7,8).
The incidence of gastrointestinal parasites in the US remains a public concern. Local cases are often linked to contaminated water, food or surfaces. Additionally, GI parasites can be acquired during international travel or by intra family transmission, with asymptomatic cases playing a role in spreading infection(9,10).
In the US, diagnosis primarily relies on microscopy, which is time-consuming, complex, labour intensive, variable in reliability, and heavily reliant on highly trained staff(11).
"Molecular detection for common gastrointestinal protozoa is the logical progression of testing, especially given the ever-increasing volumes of traditional O&P testing and the decreasing workforce and proficiency labs are experiencing." said Prof. Marc Couturier, Medical Director, Parasitology & Fecal Testing,Infectious Disease Rapid Testing, Emerging Health Crises, ARUP Laboratories, United States

There is a need for a molecular approach for gastrointestinal parasite testing for timely, sensitive and broad detection of leading clinically relevant infections
Jason's story - A case study of misdiagnosed Giardia infection
Jason had a misdiagnosed giardiasis for 60+ days from the onset of his symptoms which led to prolonged illness, inappropriate and ineffective antibiotic treatment, and financial burden. Learn about Jason's journey and how complimentary molecular diagnostic techniques support rapid and accurate detection of GI parasites.
Genetic Signatures' molecular solution for gastrointestinal parasite detection
Diagnose with Precision, Treat with Confidence
The EasyScreen™ Gastrointestinal Parasite Detection Kit and GS1 automated workflow offers a significant advancement in clinical diagnostics for expanded gastrointestinal (GI) parasite testing, providing healthcare professionals with a powerful, innovative tool for accurate and timely diagnosis.
Utilising patented 3base™ technology, this highly sensitive gastrointestinal parasite panel can identify the eight most common and clinically relevant gastrointestinal parasites in a single test, from a single patient sample.
"Genetic Signatures’ gastrointestinal parasite panel advances molecular microbiology by providing rapid diagnostics with increased sensitivity and specificity over routine methods, resulting in improved patient outcomes." Lynne Garcia, Director, LG & Associates
The automated workflow addresses the many challenges of highly manual and complex microscopic examinations. Our GS1 automated system performs sample extraction and PCR set-up, to provide a significant walkaway time and same-day reporting.
This rapid turnaround enables timely and appropriate patient management, significantly reducing healthcare costs.
Speak to your local rep. to find out more! Click HERE.
Click to learn more about the parasites we detect
Syndromic testing for 8 gastrointestinal parasites in a single test
Up to 60 patients screened in a single, automated workflow...with same day reporting!
Find out more about our unique 3base™ solution for detecting gastrointestinal parasites
More Information
References
- Hajare ST, Gobena RK, Chauhan NM, Erniso F. Prevalence of Intestinal Parasite Infections and Their Associated Factors among Food Handlers Working in Selected Catering Establishments from Bule Hora, Ethiopia. Biomed Res Int. 2021 Aug 19;2021:6669742. doi: 10.1155/2021/6669742. PMID: 34458370; PMCID: PMC8397551.
- United States Census Bureau. (2024) S and World Population Clock. https://www.census.gov/popclock/
- Sandler RS, Everhart JE, Donowitz M, et al. The burden of selected digestive diseases in the United States. Gastroenterology. 2002;122:1500–
- Amin OM. Seasonal prevalence of intestinal parasites in the United States during 2000. Am J Trop Med Hyg. 2002 Jun;66(6):799-803. doi: 10.4269/ajtmh.2002.66.799. PMID: 12224595.
- Kappus, Karl K., et al. “Results of Testing for Intestinal Parasites by State Diagnostic Laboratories, United States, 1987.” Morbidity and Mortality Weekly Report: Surveillance Summaries, vol. 40, no. SS-4, 1991, pp. 25– JSTOR, http://www.jstor.org/stable/24675438. Accessed 28 May 2024.
- Stark, D. (2023). Genetic Signatures Webinar Series: Syndromic PCR testing for GI parasites including Dientamoeba fragilis and microsporidia, and their role in gastrointestinal disease. https://geneticsignatures.com/us/resource/advances-in-gastrointestinal-parasite-testing-molecular-detection-of-gi-parasites/
- Schmidt MA, Groom HC, Rawlings AM, Mattison CP, Salas SB, Burke RM, et al. Incidence, Etiology, and Healthcare Utilization for Acute Gastroenteritis in the Community, United States. Emerg Infect Dis. 2022;28(11):2234-2242. https://doi.org/10.3201/eid2811.220247
- Sandler RS, Stewart WF, Liberman JN, Ricci JA, Zorich NL. Abdominal pain, bloating, and diarrhea in the United States: prevalence and impact. Dig Dis Sci. 2000 Jun;45(6):1166-71. doi: 10.1023/a:1005554103531. PMID: 10877233.
- Centers for Disease Control and Prevention. (2024) Traveler’s Health. https://wwwnc.cdc.gov/travel/yellowbook/2024/posttravel-evaluation/persistent-diarrhea-in-returned-travelers
- Stark, D.; Barratt, J.; Ellis, J.; Harkness, J.; Marriott, D. Repeated Dientamoeba fragilis Infections: A Case Report of Two Families from Sydney, Australia. Infect. Dis. Rep. 2009, 1, e4. https://doi.org/10.4081/idr.2009.1280
- Couturier, M. (2023). Genetic Signatures Webinar Series: The Burden of Gastrointestinal Parasites and Advances in Ova and Parasite Diagnostic Screening. https://geneticsignatures.com/us/resource/molecular-op-webinar-1/