Giardia laboratory diagnosis
Traditional O&P examinations / microscopy
There are numerous challenges associated with using microscopy to detect gastrointestinal parasites, which are common to most clinically relevant parasites that cause disease, including Giardia.
Misdiagnosis is impacted by microscopy’s low test sensitivity due to sporadic shedding of cysts into stool, which require multiple tests on alternative days to achieve >90% test sensitivity. However, most laboratories only receive a single sample due to low patient compliance. This challenge also impacts immunoassay testing methods [1].
Diagnosis of Giardiasis is through identification cysts or trophozoites in fecal samples, employing both direct mounts and concentration techniques. Cysts are commonly observed in wet mount preparations, whereas trophozoites are identifiable in permanent mounts, such as trichrome stains.
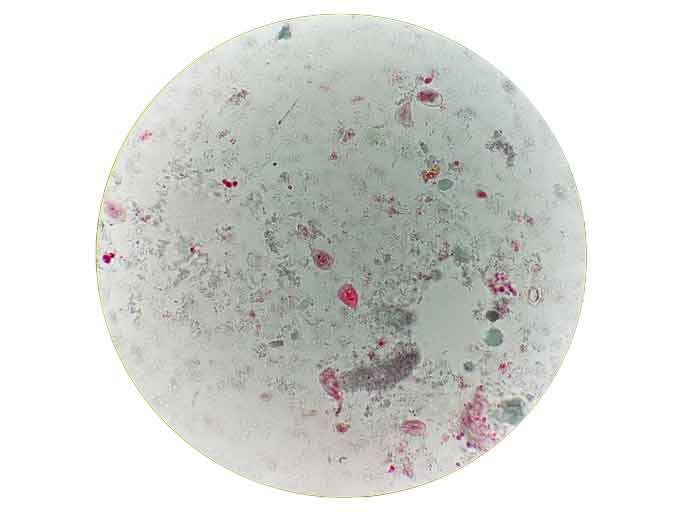
Microscopic examination of patient sample with Giardia trphozoites clearly seen. These are a teardrop shape, with linear axonemes, curved median bodies, 2 nuclei.
Fecal immunoassays
There are a number of immunoassays that can detect Giardia in stool.
Immunoassays are also reliant on two stool samples from each patient to confirm a negative result for Giardia infection.
Immunoassays include:
Direct immunofluorescence assay (DFA) - Antibodies tagged with fluorescent markers are added to stool and incubated. Visualization under a fluorescent microscope shows the Giardia cysts as green, glowing ovoid objects.
Enzyme immunoassay (EIA) - Does not rely on microscopy and is useful for screening large numbers of specimens. Borderline positives and questionable negatives obtained with this technique should be further confirmed by DFA. Antigens of Giardia are detected in the feces using this method; therefore, specimens should not be concentrated prior to testing.
Rapid immunochromatographic cartridge assays - May be used with preserved specimens and are quick and easy to perform. Antigens of Giardia are detected in the feces using this method; therefore, specimens should not be concentrated prior to testing. Borderline positives and questionable negatives obtained with this technique should be further confirmed by DFA.
Molecular methods for detecting Giardia lamblia
Detection of Giardia DNA/RNA in feces is commonly available in lab-developed tests and a number of commercially available gastrointestinal pathogen screening assays.
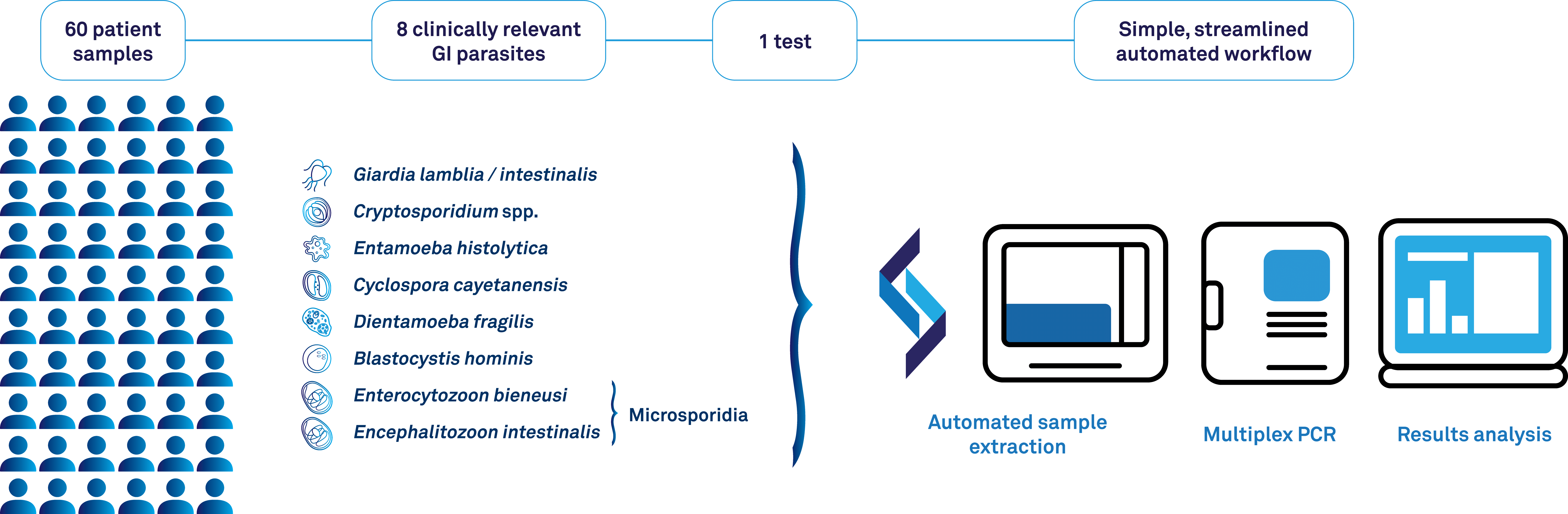
There are many advantages for employing molecular methods for detecting Giardia lamblia with Genetic Signatures' EasyScreen™ Parasite Detection Kit and automated GS1 workflow. This includes the ability to screen for 7 other leading gastrointestinal parasites within the one test, supporting accurate identification of causative agents of parasite-associated gastrointestinal infection.
Syndromic testing for gastrointestinal parasites also supports the identification of unsuspected cases or undiagnosed cases. This is particularly important for patients with variable symptoms.
Syndromic testing for 8 gastrointestinal parasites in a single test
Up to 60 patients screened in a single, automated workflow...with same day reporting!