Entamoeba histolytica (true pathogen) laboratory diagnosis
Traditional O&P examinations / microscopy
There are numerous challenges associated with using microscopy to detect gastrointestinal parasites, which are common to most clinically relevant parasites that cause disease, including Blastocystis hominis.
Misdiagnosis is impacted by microscopy’s low test sensitivity (<50%) due to sporadic shedding of cysts into stool, which require multiple tests on alternative days to achieve >90% test sensitivity. However, most laboratories only receive a single sample due to low patient compliance. This challenge also impacts immunoassay testing methods [1].
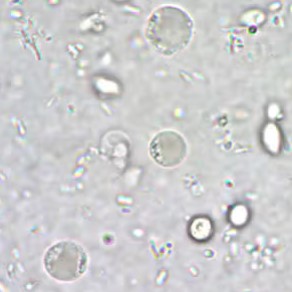
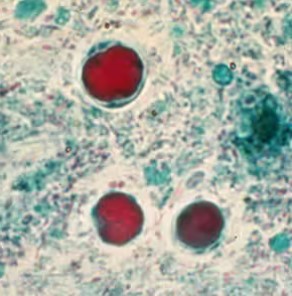
Permanently stained slides are preferred over unstained wet-mount preparations, as the parasite is difficult to detect using wet mounts only. In addition, in wet mounts, fecal debris may be mistaken for Blastocystis spp. and vice versa [2].
Reporting of Blastocystis spp. can be problematic, as the pathogenic subtypes cannot be differentiated from non-pathogenic, when using microscopy.
Fecal immunoassays
No immunoassays are commercially available.
*Image credit: CDC website - www.cdc.gov/dpdx/blastocystis
Blastocystis parasite morphology is round and surrounded by multiple small nuclei about 6-40 µm in size.
Molecular methods for detecting Blastocystis
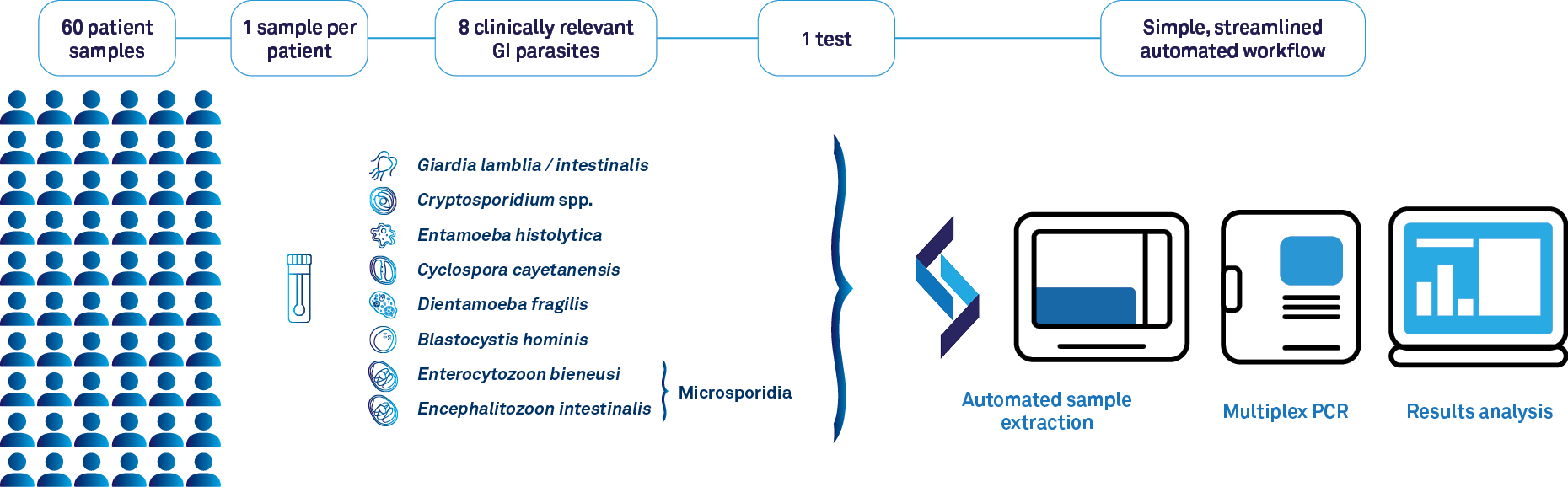
There are many advantages for employing molecular methods for detecting Blastocystis hominis. This includes the ability to screen for 7 other leading gastrointestinal parasites within the one test, supporting accurate and definitive identification of causative agents of parasite-associated gastrointestinal infection.
Syndromic testing for gastrointestinal parasites also supports the identification of unsuspected cases or undiagnosed cases. This is particularly important for patients with variable symptoms.
Screening of donor fecal microbiota transplantation using a molecular parasite panel would ensure that asymptomatic gastrointestinal parasite infections are detected.
Currently, Genetic Signatures EasyScreen Gastrointestinal Detection Kit is the only FDA (510k) cleared molecular diagnostic solution available that can detect Blastocystis hominis.
Genetic Signature's molecular workflow for GI parasite detection
Up to 30 patients, one sample, one test, for the detection of 8 leading GI parasites!
References
- Hiatt RA, Markell EK, Ng E. How many stool examinations are necessary to detect pathogenic intestinal protozoa? The American Journal of Tropical Medicine and Hygiene. 1995 Jul;53(1):36-39. PMID: 7625530.
- CDC DPDx - Laboratory Identification of Parasites of Public Health Concern. Blastocystis sp. www.cdc.gov/dpdx/blastocystis/index.html


