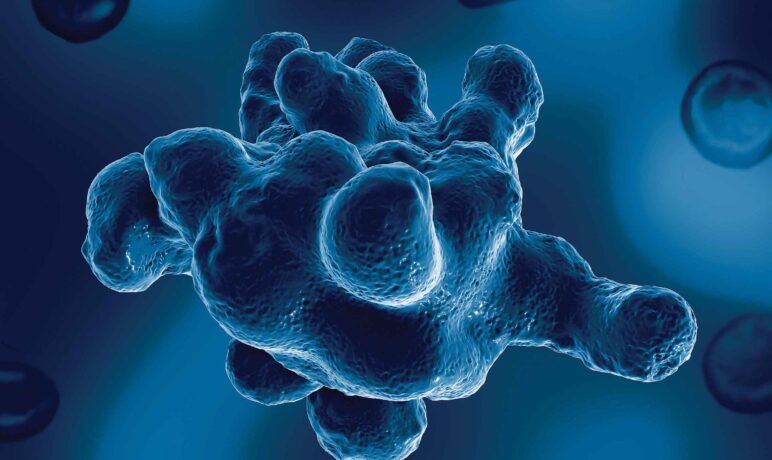
Most Entamoeba infections in humans are caused by non-pathogenic species such as Entamoeba dispar, Entamoeba moshkoviskii and Entamoeba coli. The only amoebic species that causes disease in humans is Entamoeba histolytica (true pathogen).
E. histolytica is the causative agent of amoebiasis and is responsible for dysentery and liver abscesses with invasive disease developing in approximately 10% of infected individuals. The burden of amoebiasis is difficult to determine due to many factors including the limited diagnostic and surveillance capacities in endemic regions, such as Central and South America, Africa, and Asia [1]. Worldwide, it has been estimated that up to 50 million people are affected by E. histolytica, primarily in developing countries, and it is responsible for over 100,000 deaths a year which ranks only below malaria with regard to patient mortality [1].
E. histolytica is also present in industrialised countries but generally confined to returning travellers or immigrants from endemic countries. E. histolytica is typically transmitted to the population by the ingestion of infected water or food contaminated with faecal material, faecal-oral transmission within households and men who have sex with men [2].
Amebiasis: symptoms, prevention & treatment
Patients infected with E. histolytica (true pathogen) can be asymptomatic or experience a wide range of symptoms.
Learn more here
E. histolytica laboratory diagnosis
Learn more about the challenges of traditional techniques for detecting E. histolytica (true pathogen), and the benefits of molecular testing.
Genetic Signatures' molecular solution for GI parasite testing
A comprehensive FDA 510(k) approved molecular solution for detecting gastrointestinal parasites
Syndromic testing for 8 gastrointestinal parasites in a single test
Up to 60 patients screened in a single, automated workflow...with same day reporting!
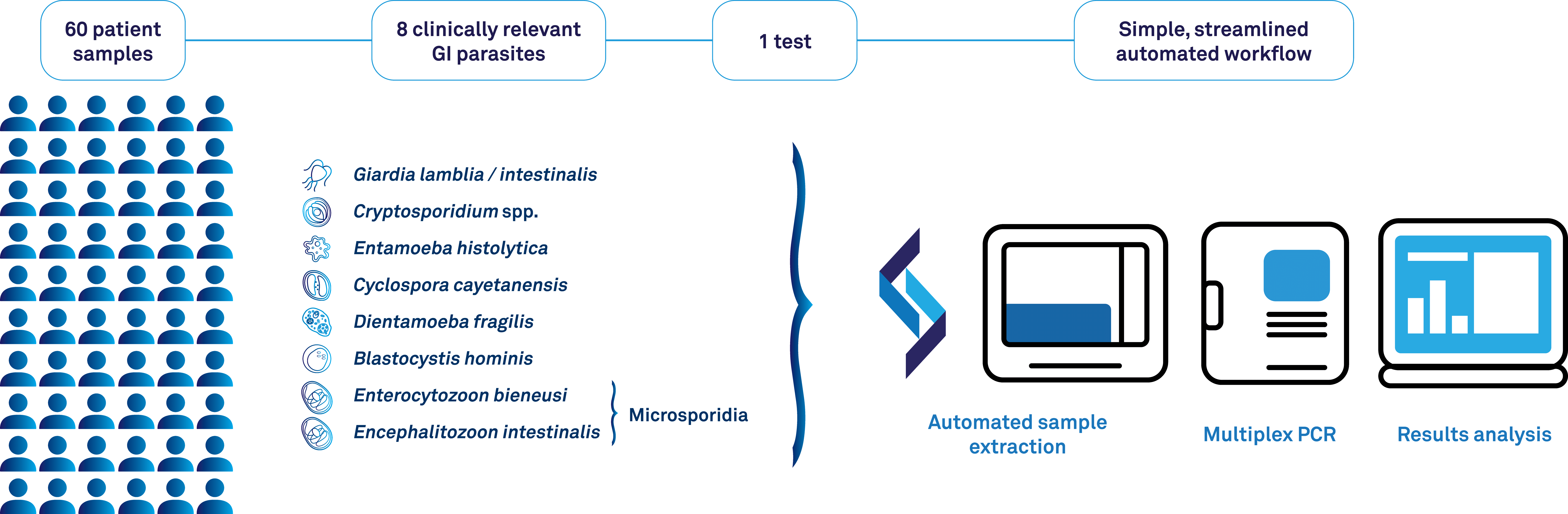
Find out more about our unique 3base™ solution for detecting gastrointestinal parasites
Click to learn more about the parasites we detect
More Information
References
- Kantor M, Abrantes A, Estevez A, Schiller A, Torrent J, Gascon J, Hernandez R, Ochner C. Entamoeba Histolytica: Updates in Clinical Manifestation, Pathogenesis, and Vaccine Development. Can J Gastroenterol Hepatol. 2018 Dec 2; 2018:4601420. doi: 10.1155/2018/4601420. PMID: 30631758; PMCID: PMC6304615.
- Lo YC, Ji DD, Hung CC. Prevalent and incident HIV diagnoses among Entamoeba histolytica-infected adult males: a changing epidemiology associated with sexual transmission--Taiwan, 2006-2013. PLoS Negl Trop Dis. 2014 Oct 9;8(10):e3222. doi: 10.1371/journal.pntd.0003222. PMID: 25299178; PMCID: PMC4191956.