Dientamoeba fragilis laboratory diagnosis
Diagnostic challenges
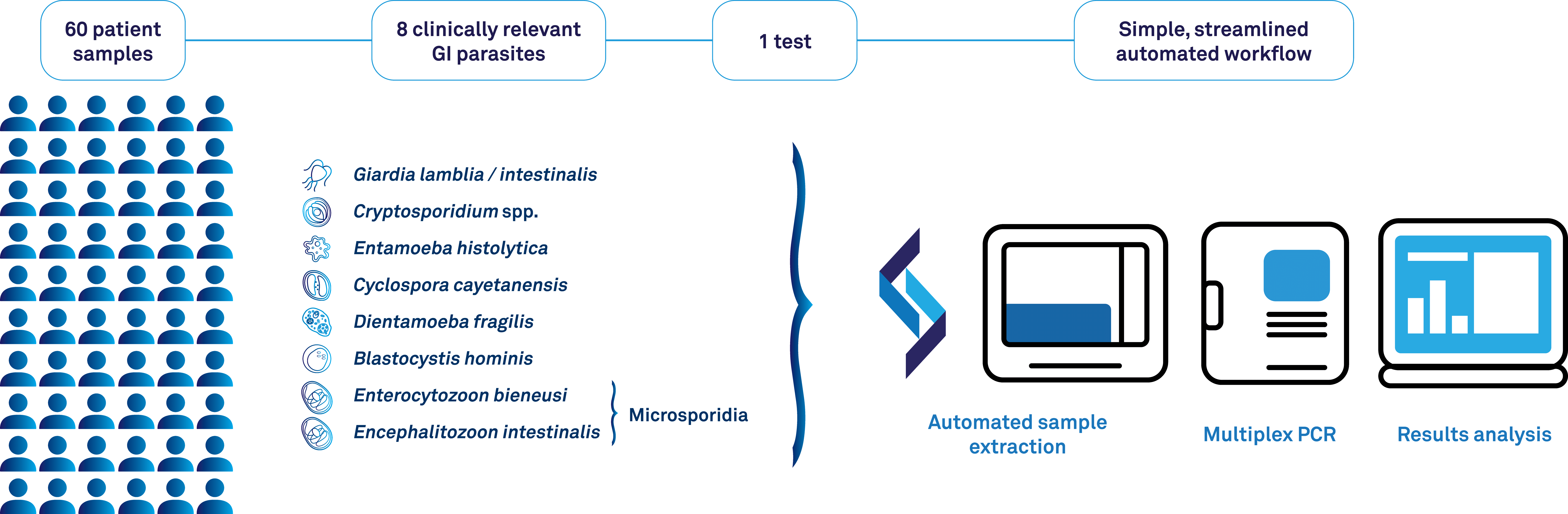
The difficulty involved in microscopically diagnosing D. fragilis in feces has led to the development of real-time PCR methodologies for the detection of D. fragilis in stool samples. Genetic Signatures' EasyScreen™ Gastrointestinal Parasite Detection Kit and GS1 automated workflow can detect D. fragilis, and 7 other leading parasites, in a single test.
This solution has received FDA 510(k) clearance for sales in the United States.
See below for details.
Traditional O&P examinations / microscopy
Diagnosis of D. fragilis has traditionally relied upon microscopy of fixed faecal smears. Because of the “fragile” nature of the organism, prompt fixation of clinical specimens is essential as the trophozoites degenerate rapidly once passed in stool samples.
The trophozoite stage of the parasite is not usually detectable if stool concentration methods are used. In addition, D. fragilis trophozoites can easily be overlooked or misidentified because they are pale-staining and their nuclei sometimes resemble those of Endolimax nana or Entamoeba hartmanni.
The trophozoites are also highly pleomorphic, with many variations in shape and size making diagnosis by microscopy difficult. It is almost impossible to identify this parasite using a wet mount microscopic exam, thus, a permanent stain is mandatory.
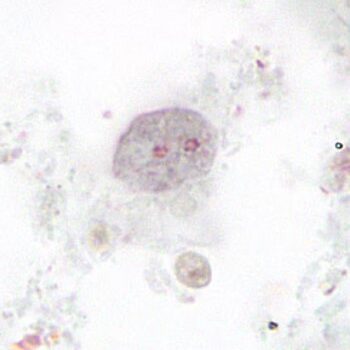
Fecal immunoassays
No immunoassays are commercially available.
*Image credit: CDC website - www.cdc.gov/dpdx/dientamoeba
Molecular methods for detecting D. fragilis
Currently, Genetic Signatures EasyScreen™ Gastrointestinal Detection Kit is the only FDA 510(k) cleared molecular diagnostic solution available that can detect Dientamoeba fragilis, together with 7 other leading gastrointestinal parasites, in a single test.
Syndromic testing for gastrointestinal parasites also supports the identification of unsuspected cases or undiagnosed cases. This is particularly important for patients with variable symptoms.
Screening of donor fecal microbiota transplantation using a molecular parasite panel would ensure that asymptomatic gastrointestinal parasite infections are detected.
Syndromic testing for 8 gastrointestinal parasites in a single test
Up to 60 patients screened in a single, automated workflow...with same day reporting!
Find out more about our unique 3base™ solution for detecting gastrointestinal parasites
More Information
Click to learn more about the parasites we detect
References