Cryptosporidium laboratory diagnosis
Traditional O&P examinations / microscopy
There are numerous challenges associated with using microscopy to detect gastrointestinal parasites, which are common to most clinically relevant parasites that cause disease, including Crytosporidium spp.
Misdiagnosis is impacted by microscopy’s low test sensitivity (<50%) due to sporadic shedding of cysts into stool, which require multiple tests on alternative days to achieve >90% test sensitivity. However, most laboratories only receive a single sample due to low patient compliance. This challenge also impacts immunoassay testing methods [1].
Modified acid-fast staining methods, with or without stool concentration, are most frequently used in clinical laboratories, as this parasite is very difficult to identify using wet mount slides only. In addition, routine trichrome staining will not detect these parasites.
Immunofluorescence microscopy has higher sensitivity and specificity for microscopic methods.
Fecal immunoassays
Direct Immunofluorescence Assay (DFA) - Stool samples are treated with antibodies labeled with fluorescent markers and is considered the gold standard.[6]
Enzyme Immunoassay (EIA) – This method tests for antigens on the surface of Cryptosporidium and is ideal for screening large sample volumes. Uncertain or borderline results should be validated with DFA. Specimen concentration should not be performed prior to testing for antigens.
Rapid Immunochromatographic Cartridge Assays – These antigen assays are quick and simple to use. Other parasite such as Giardia and Entamoeba histolytica may also be tested with Cryptosporidium. Uncertain or borderline results should be validated with DFA. Specimen concentration should not be performed prior to testing for antigens.
Molecular methods for detecting Cryptosporidium
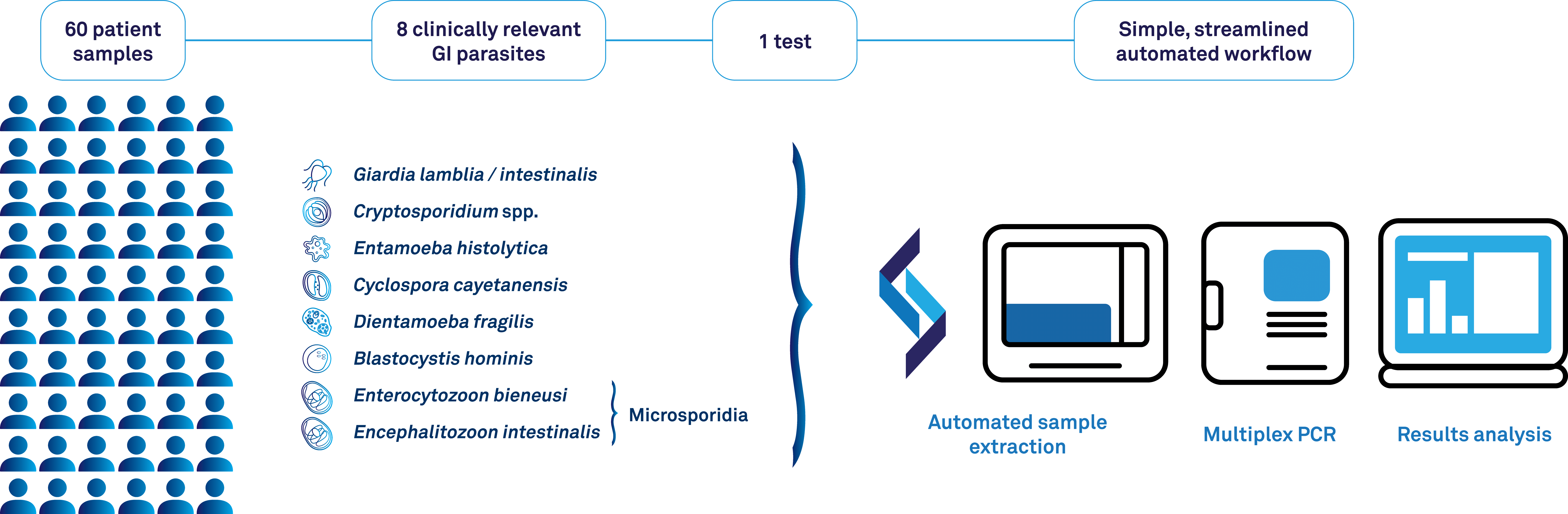
Currently, Genetic Signatures EasyScreen Gastrointestinal Detection Kit is the only FDA 510(k) cleared molecular diagnostic solution available that can detect Cryptosporidium spp. with 7 other leading gastrointestinal parasites.
Molecular parasite testing supports accurate and definitive identification of causative agents of parasite-associated gastrointestinal infection. The advantage of this solution is high test sensitivity and specificity, without the need for highly trained molecular biologists. Also, infections can be detected even in the absence of clinical suspicion.
This molecular solution also eliminates the need for laboratories to employ complex staining processes to identify this parasite, saving time and cost for the laboratory.
60 patient samples can be screening in a single workflow, only requiring a single patient sample to achieve high test sensitivity (rather than 3). 8 parasites are targeted in a single test, employing an automated workflow to further enhance laboratory productivity. Same-day results are available, instead of days or weeks required for traditional diagnostic processes.
Syndromic testing for 8 gastrointestinal parasites in a single test
Up to 60 patients screened in a single, automated workflow...with same day reporting!
Find out more about our unique 3base™ solution for detecting gastrointestinal parasites
References
- Hiatt RA, Markell EK, Ng E. How many stool examinations are necessary to detect pathogenic intestinal protozoa? The American Journal of Tropical Medicine and Hygiene. 1995 Jul;53(1):36-39. PMID: 7625530.
- CDC DPDx - Laboratory Identification of Parasites of Public Health Concern. Blastocystis sp. www.cdc.gov/dpdx/blastocystis/index.html