Cyclospora cayetanensis is a coccidian parasite that causes cyclosporiasis. Like other coccidian parasites, infected people shed oocysts (rather than cysts or ova) in their feces. These oocytes are often non-infectious when initially excreted. To become infectious, the oocysts mature in favourable environmental conditions. This process takes up to 14 days so it’s unlikely that Cyclospora can spread directly between people [1].
C. cayetanensis is the only species known to infect humans and has been reported worldwide, in both developed and developing countries, though is most prevalent in tropical and subtropical areas [2], and in settings with poor sanitation where the environment is contaminated with human faeces from infected individuals.
Transmission is generally food and waterborne and associated with contaminated fresh produce such as raspberries, blackberries, strawberries, blueberries, basil, and ready-to-eat bagged salads [2].
Though have been several large outbreaks of food-associated illness in the US, though infection is more frequently seen as sporadic individual cases and small clusters of illness.
Cyclospora cases mainly occurr between May and July in the US, though seasonality is also seen worldwide, which may be affected by rain, temperature, and humidity [3].
Cyclospora symptoms, prevention & treatment
Patients infected with Cyclospora cayetanensis can experience a wide range of symptoms.
Cyclospora laboratory diagnosis
Learn about the challenges of traditional diagnostic techniques for detecting Cyclospora infection, and the benefits of molecular testing
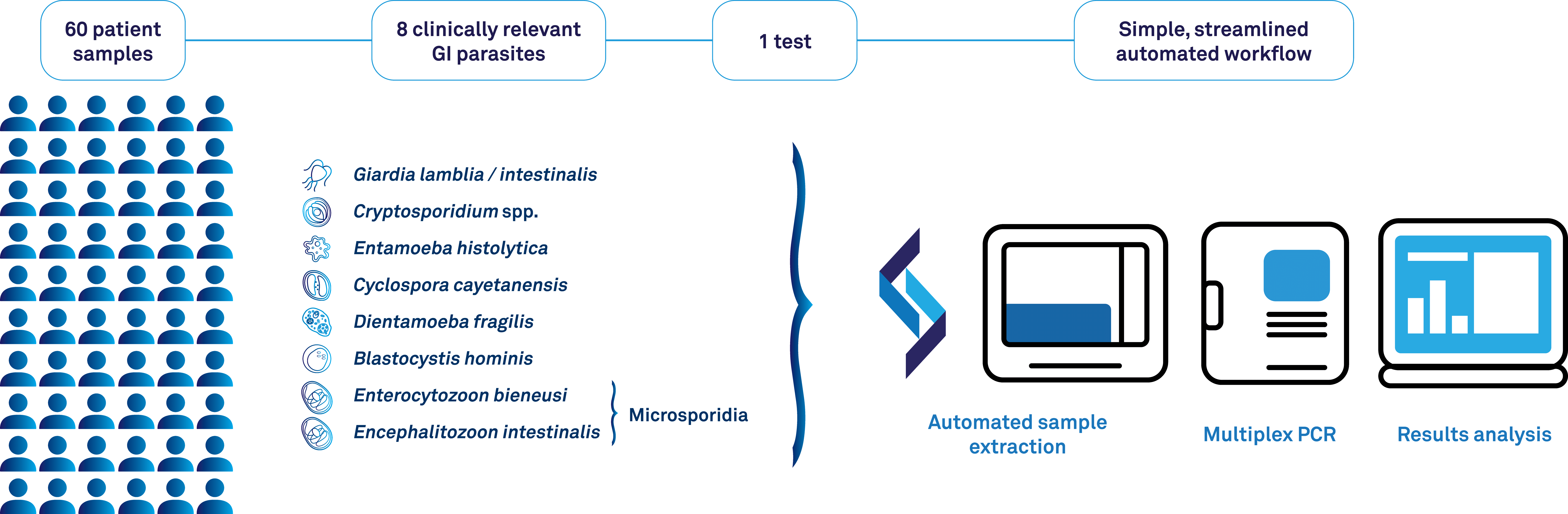
Genetic Signatures' molecular solution for GI parasite testing
A comprehensive FDA 510(k) approved molecular solution for detecting gastrointestinal parasites
Syndromic testing for 8 gastrointestinal parasites in a single test
Up to 60 patients screened in a single, automated workflow...with same day reporting!
Find out more about our unique 3base™ solution for detecting gastrointestinal parasites
References
- FDA https://www.fda.gov/food/foodborne-pathogens/bad-bug-book-second-edition
- Almeria S, Cinar HN, Dubey JP. Cyclospora cayetanensis and Cyclosporiasis: An Update. Microorganisms. 2019 Sep 4;7(9):317. doi: 10.3390/microorganisms7090317. PMID: 31487898; PMCID: PMC6780905.
- CDC DPDx-Laboratory Identification of Parasites of Public Health Concern. Cyclosporiasis. www.cdc.gov/dpdx/cyclosporiasis/index.html