OUR TECHNOLOGY
Our technology is designed to identify and simplify
The technology not only serves to identify methylation patterns in the
DNA, it can also be used to simplify DNA sequences more generally,
particularly in lower organisms.
ABOUT US
Transforming Molecular Diagnostics
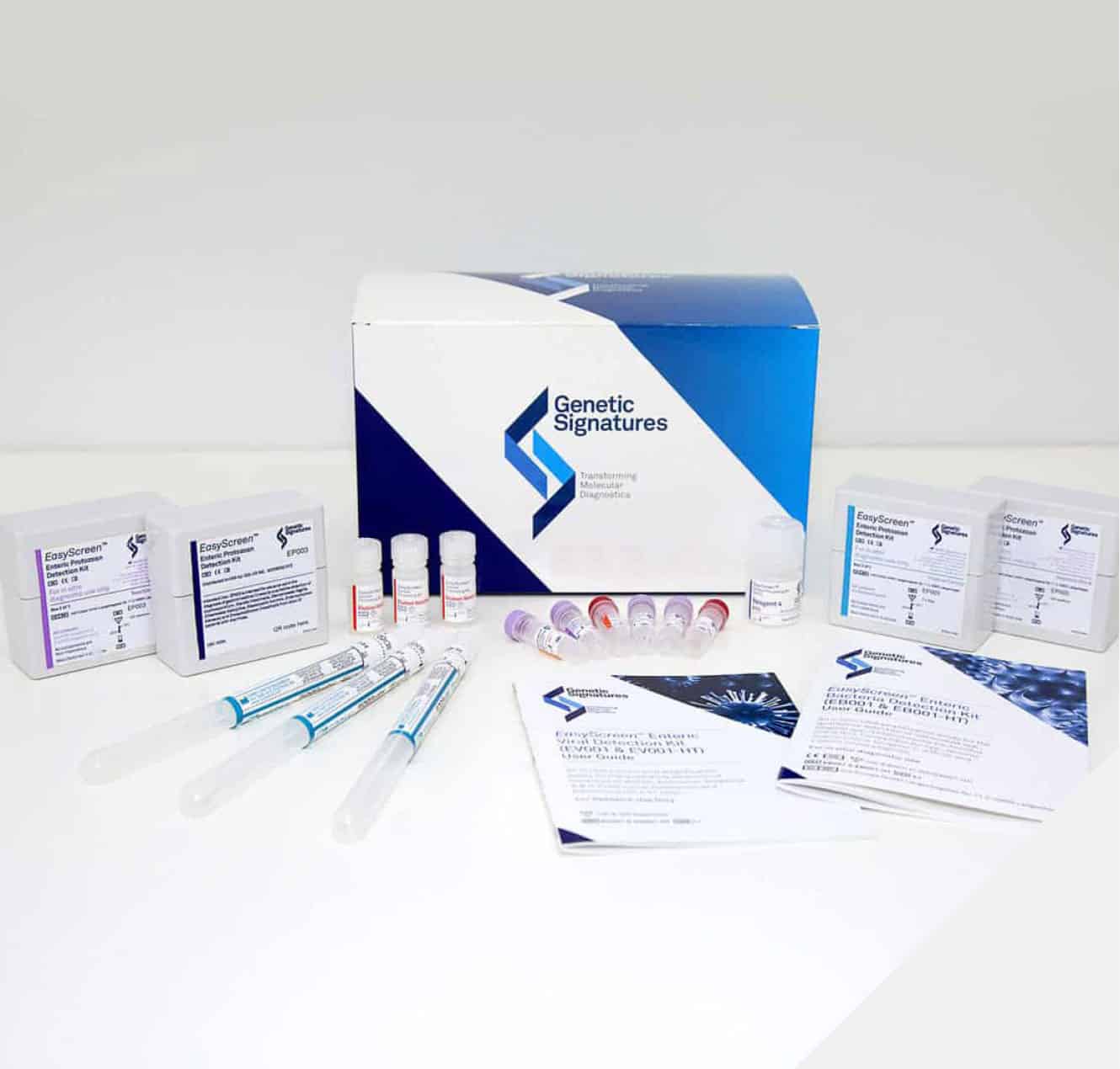
We focus on the development and commercialisation of our proprietary platform technology, 3base™. Genetic Signatures has released a suite of real-time PCR based products for the routine detection of infectious diseases under the EasyScreen™ brand.


We're driving innovation together
Our Products
Genetic Signatures is transforming molecular diagnostics via
streamlined sample processing methods linked to highly multiplexed
real-time PCR screening assays.
The EasyScreen™ Detection Kits simultaneously detect a larger
number of pathogen targets in a shorter time than conventional
methods.
Media Centre
Why invest with Genetic Signatures? We're at the forefront of Specialist Molecular Diagnostics.
Find investor resources and ASX announcements here.
