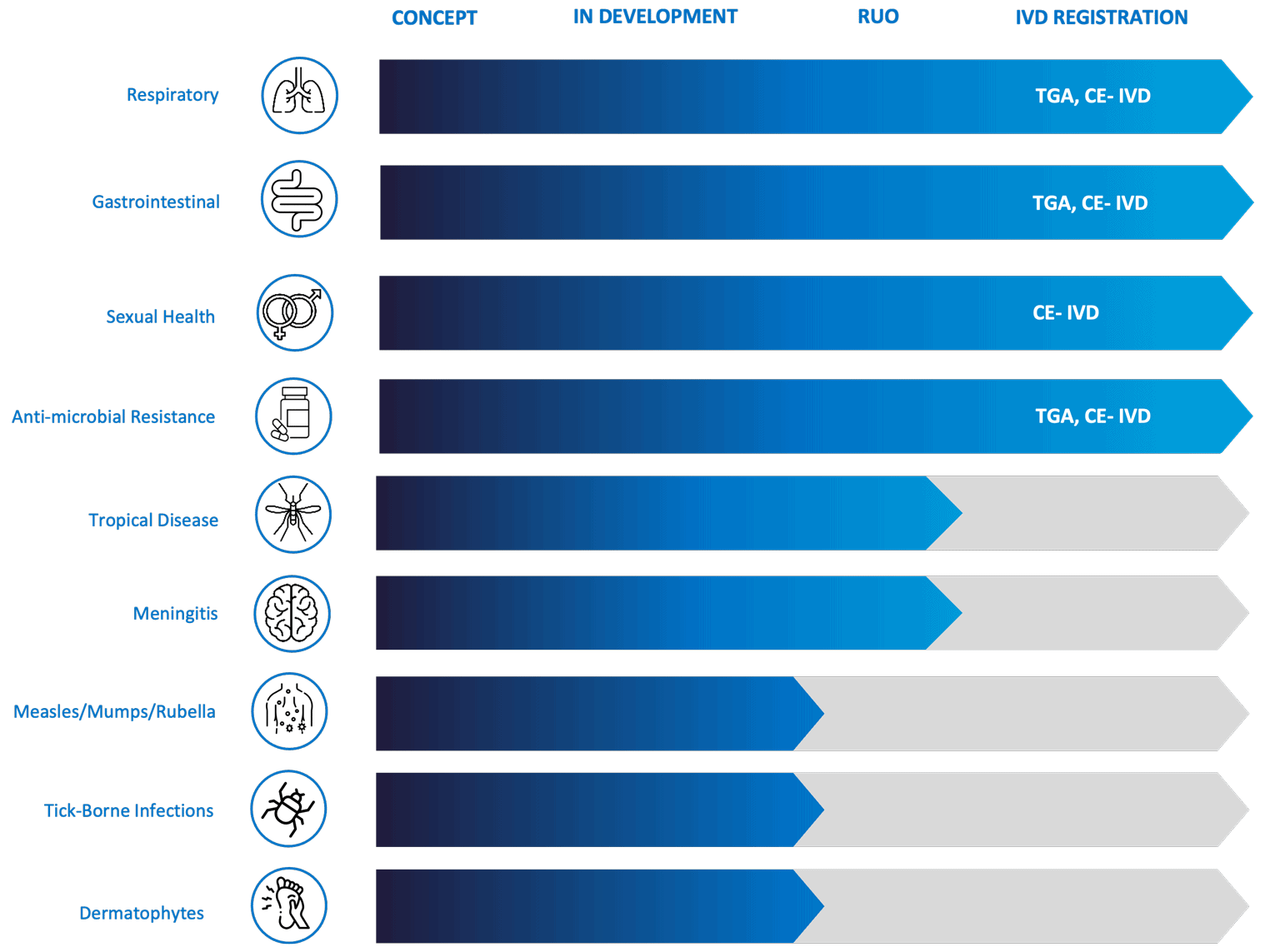
Robust product pipeline & research use only products available
Genetic Signatures has an extensive product pipeline with the diagnostic menu expanding further. More than 5 new syndromic products are currently in development. The company is also focused on the IVD registration of existing research use only (RUO) products to bring more syndromic tests to diagnostic laboratories.
For more information on our products in the pipeline contact your local representative.

